Q&A with Dr. Egilius L.H. Spierings
Dr. Spierings currently serves as both medical director and principal investigator at MedVadis Research, as well as director of Boston Headache Institute at Boston Advanced Medicine.

Dr. Egilius L.H. Spierings founded MedVadis Research—then known as the Clinical Headache Research Center—in 1994. Having dedicated the last four decades of his career to advancing patient care through clinical trials, he currently serves as both medical director and principal investigator at MedVadis Research, as well as director of Boston Headache Institute at Boston PainCare.
Q: How has clinical trial research evolved over the course of your career?
A: The clinical trial process has changed dramatically. When I first started in 1980, clinical trials were conducted by pharmaceutical sales representatives who visited physicians in their offices. There were no protocols, no source documents, no informed consent—just blinded trial medication (which could be placebo) and a paper diary. Since then, the process of conducting clinical trials has become very regulated and digitized. It’s been exciting to watch the field evolve and see how the changes help us to make a greater impact.
Q: What has MedVadis Research’s work accomplished?
A: From the start, our goal has been to improve lives through the advancement and discovery of new medical treatments through clinical research. I’m proud to say that we’ve seen our hard work pay off in many ways. In the last three years, our migraine clinical trials alone have resulted in no less than 10 medications approved by the FDA for the abortive or preventive treatment of migraines. In addition to playing a role in bringing new treatments to market for general use, we’ve also helped countless individuals who have come through our facility as trial participants. It’s extremely rewarding to have that kind of impact on our local community.
Q: What’s next for MedVadis Research?
A: We’ve recently embraced Alzheimer’s clinical trial research–an area of practice that could benefit millions of patients. We’ve already completed enrollment for two trials and are preparing to commence enrollment for several more. The quest for an effective, safe, and well-tolerated medication to slow or stop the progression of Alzheimer’s disease and, hopefully, an eventual treatment to reverse its consequences. It’s an exciting time, and we, at MedVadis, hope to contribute to this research as much as we have contributed to the migraine therapeutic area.
Q: For decades MedVadis Research has focused on chronic pain and migraine. Why Alzheimer’s, and why now?
A: It’s something we’ve been considering for a while, but our resources were focused on other areas. Now, Alzheimer’s clinical trial research has matured to the extent that antibodies are being investigated. Because we have experience with some of these proteins in the context of migraines (CGRP antibodies) and chronic pain (NGF antibodies), it makes sense to add these trials to our portfolio. We’re pleased to have the opportunity to help improve care for a whole new segment of patients.
Q: What role do study volunteers play in this process?
A: Our volunteers are invaluable. For a medication to be approved for marketing and general use, the FDA requires at least two randomized, double-blind, placebo-controlled trials, confirming efficacy. In addition, at least 300 patients must be exposed to the medication for 6 months and at least 100 patients exposed for 12 months for tolerability and safety documentation. Volunteers who partner with MedVadis help satisfy these requirements, which may ultimately enable physicians and other healthcare providers to prescribe new medications, benefiting the health and wellbeing of patients around the world.
Ready to Participate?
Take a look at our current trials or join our mailing list to find the right study for you.
Together, we can advance medical treatments for generations.

What is a memory screening?
If you are not worried about your memory, you may not have considered participating in a memory screening.
Diabetic neuropathy: Current treatment options & clinical research
Diabetic neuropathy—or nerve damage—is a common and serious complication of diabetes.
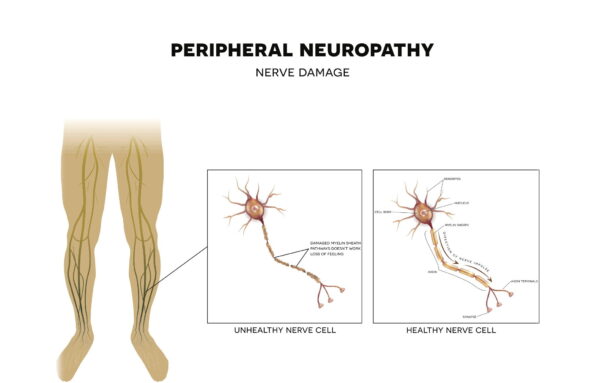
What you need to know about diabetic peripheral neuropathy
Diabetic peripheral neuropathy is a type of nerve damage that affects approximately 10 million Americans.
