Working to make new treatments available.
Get involved and help advance care.
Together, we can work to understand common health issues and develop new solutions
What we do
MedVadis Research at Boston Advanced Medicine is a clinical trial research center founded in 1996 by Egilius L.H. Spierings, M.D., Ph.D. Since its inception, MedVadis has been dedicated to the advancement and discovery of new medical treatments through clinical research. Over the past 25 years, the center has conducted more than 300 clinical research trials involving a range of medical conditions, including migraine, Alzheimer’s disease, Parkinson’s disease, chronic low-back pain, osteoarthritis, painful diabetic neuropathy, fibromyalgia, asthma, hypertension, ulcerative colitis, irritable bowel disease, and diabetes.


Who we are
In October 2019, MedVadis Research merged with Boston Advanced Medicine, a state-of-the-art center for the treatment of chronic pain and headaches. This merger allowed MedVadis to extend the opportunity for research participation to more individuals in our local community, while also offering greater access to chronic pain and headache treatment to those in need of such services. Dr. Spierings currently serves as the center’s medical director and along with Dr. David DiBenedetto, is one of the principal investigators. Our facility is located just outside Boston, in Waltham, Massachusetts.
our blog
See what’s happening at MedVadis
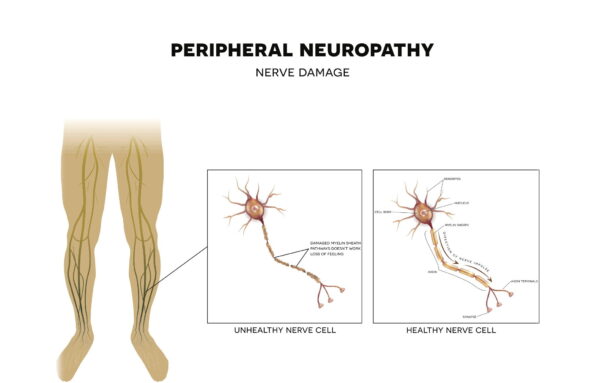
What you need to know about diabetic peripheral neuropathy
Diabetic peripheral neuropathy is a type of nerve damage that affects approximately 10 million Americans.
Diabetic neuropathy: Current treatment options & clinical research
Diabetic neuropathy—or nerve damage—is a common and serious complication of diabetes.

